Analytical Reference substance
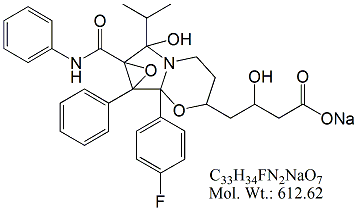
Atorvastatin FX1 Impurity
1b-(4-Fluorophenyl)hexahydro-?,7-dihydroxy-7-(1-methylethyl)-1a-phenyl-7a-[(phenylamino)carbonyl]-3H-oxireno[3,4]pyrrolo[2,1-b][1,3]oxazine-3-butanoic acid sodium salt ; (3R)-4-(1b-(4-Fluorophenyl)-7-hydroxy-7-isopropyl-1
Product Number: OLY-ATOR-39
CAS Number: 1315629-79-8 (sodium salt) ; 873950-18-6 (acid)
Lot Number:
Molecular Formula: C33H34FN2NaO7
Molecular Weight: 612.62
Long Term Storage: 02-08 °C
Appearance:
Melting Point:
Purity By HPLC: NLT 95%
| Sr No. | Test | Results | Date ref. |
|---|---|---|---|
| 1 | 1H NMR | Confirm | Report Attached |
| 2 | Mass | Confirm | Report Attached |
| 3 | HPLC | NLT 95% | Report Attached |
This certificate is valid for two years from the date of Manufacturing Provided the substance is stored under the recommended conditions.
Document data reference:
Prepared by
Dr. Ashish Keche
Head of QA & QC
Dr. Ashish Keche
Head of QA & QC
Reviewed by
Dr. Girish Hatnapure
Head of R& D
Dr. Girish Hatnapure
Head of R& D
Approved by
Dr. Atish Rodge
Managing Director
Dr. Atish Rodge
Managing Director
Corporate Office: Unit No F/10, 1st Floor Shubha Parvti Industrial Premises, Dombivali (E) Thane-421204 www.olympusimpuritiesstandard.com, +91-7506256625, Info@olympusimpuritiesstandard.com